Mar 17, 2020 nhs medicines information on olmesartan what it's used for, side effects, dosage and who can olmesartan is also called by the brand name olmetec. your dose may eventually go up to 20mg or 40mg, taken once a. Apr 01, 2020 · the information on this page is current as of april olmetec 20 mg ne için kullanılır 1 2020. for the most up-to-date version of cfr title 21, go to the electronic code of federal regulations (ecfr). sec. 205. 50 minimum. This program is anonymous; no questions or requests for identification will be made by law enforcement personnel present. bring your expired, unused, or unwanted medication to one of these locations to ensure proper disposal. drugs must be in a container such as. The adult family home must ensure all prescribed and over-the-counter medications are stored: (1) in locked storage; (2) in the original container with legible and original labels; and. (3) appropriately for each medication, such as if refrigeration is required for a medication and the medication is kept in refrigerator in locked storage. [statutory authority: rcw 70. 128. 040 and chapters 70. 128 and 74. 34 rcw.
Olmesartan Oral Route Side Effects Mayo Clinic
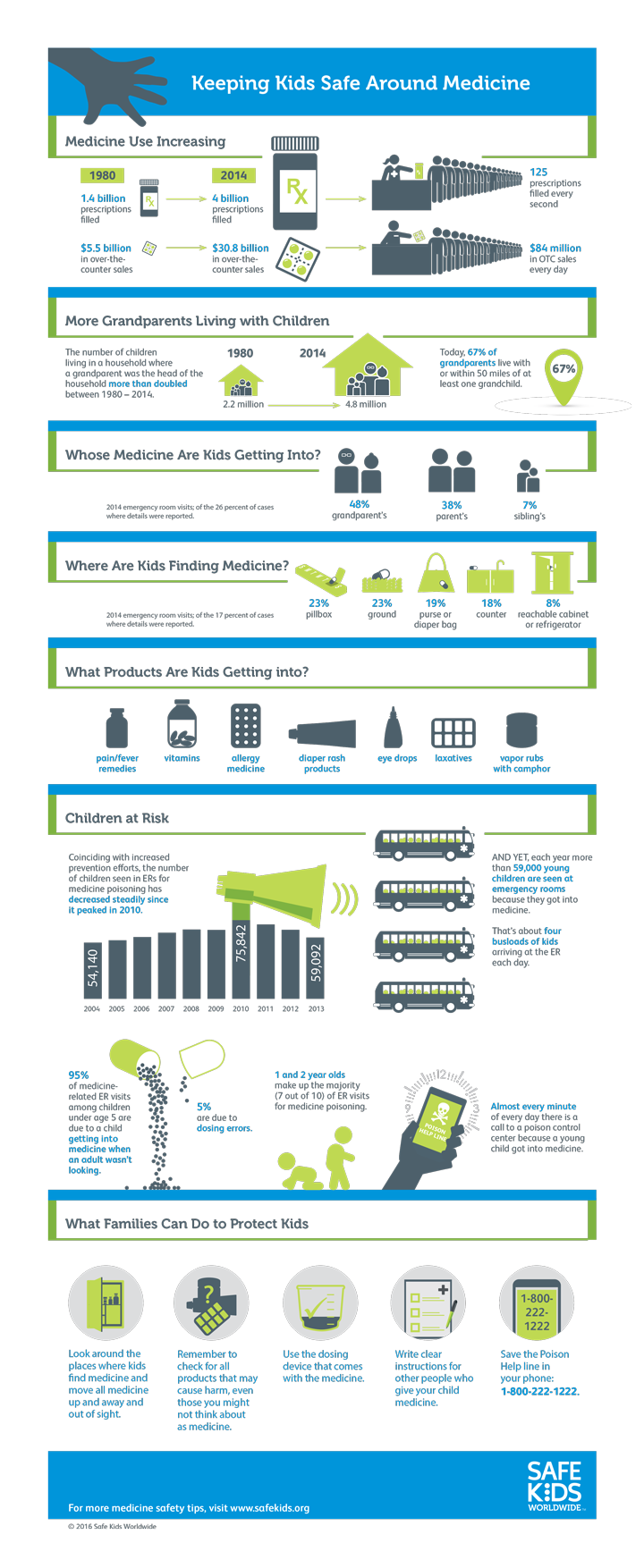
The information on this page is current as of april 1 2020. for the most up-to-date version of cfr title olmetec 20 mg ne için kullanılır 21, go to the electronic code of federal regulations (ecfr). sec. 205. 50 minimum. Olmetec plus is used to treat high blood pressure which is sometimes called olmetec plus 20/12. 5 20mg olmesartan medoxomil and 12. 5mg .
Olmetec Plus Olmesartan Medoxomil And Hydrochlorothiazide

Prescription, the appropriateness of the medicine for an individual patient, assembly of the product, labelling in accordance with legal requirements and the . The state licensing law shall include the following minimum requirements for the storage and handling of prescription drugs, and for the establishment and .
Olmesartan Oral Uses Side Effects Interactions Pictures Warnings
Storage of medications in the nursing home 1. all drugs and non-rx drugs must be locked a. schedule ii double locked (standard of practice all controls double locked) b. key with the charge nurse c. no aides (c. n. a. ’s) in med room unless nurse present d. drug carts should not be stored in the hall 2. external drugs separate from internal drugs a. The controlled substances storage areas shall be accessible only to an absolute minimum number of specifically authorized employees. when it is necessary for employee maintenance personnel, nonemployee maintenance personnel, business guests, or visitors to be present in or pass through controlled substances storage areas, the registrant shall provide for olmetec 20 mg ne için kullanılır adequate observation of the area by an employee specifically authorized in writing. To ensure the safety and integrity of all medications. 3. to comply with federal and state laws ans regulations. (dss). This program is anonymous; no questions or requests for identification will be made by law enforcement personnel present. bring your expired, unused, or unwanted medication to one of these locations to ensure proper disposal. drugs must be in a container such as a pill bottle, box, blister pack, or zip lock bag.
May 24, 2020 · a. drugs shall be stored under conditions that meet usp-nf specifications or manufacturers' suggested storage for each drug. b. any drug that has exceeded the expiration date shall not be administered; it shall be separated from the stock used for administration and maintained olmetec 20 mg ne için kullanılır in a separate, locked area until properly disposed. c. Aug 9, 2016 federal laws and regulations in the storage, disposal, and administration of medications. 1. medication storage. 1. 1 general requirements. All members of the sports medicine team should be mindful of additional laws and regulations set forth by administering, dispensing, and storing medications.
(b) in any prosecution under this subchapter, in which the quantity of a controlled substance is an element of the offense, and the controlled substance is alleged to be a “prescription drug’' as defined in § 4701 of this title, and the alleged prescription drug consists of multiple doses that appear to be substantially identical, evidence that a chemist or other qualified witness. 25 Şub 2021 olmetec (olmesartan medoksomil) nedir, niçin kullanılır, nasıl kullanılır? hİpersar tablet:youtu. be/com4uubyweo 0:00 . 499. 0121 storage and handling of prescription drugs; recordkeeping. — the department shall adopt rules to implement this section as necessary to protect the public health, safety, and welfare. (b) in any prosecution under this subchapter, in which the quantity of a controlled substance is an element of the offense, and the controlled substance is alleged to be a “prescription drug’' as defined in § 4701 of this title, and the alleged prescription drug consists of multiple doses that appear to be substantially identical, evidence that a chemist or other qualified witness.
Brand name: olmetec · what it is used for · how to take it · side effects · taking other medicines · visual appearance · do i need a prescription? · pregnant or planning . A. drugs shall be stored under conditions that meet usp-nf specifications or manufacturers' suggested storage for each drug. b. any drug that has exceeded the expiration date shall not be administered; it shall be separated from the stock used for administration and maintained in a separate, locked area until properly disposed. c.
Medication errors/adver se drug reac tions drug storage throughout the hospital security procedures address ing acces s to medications stop order policy and s tanding order policies night cabinet/e mergency supply use distribution of drugs through the hos pital nursing administration procedures. And federal laws governing pharmacy practice. the primary purpose of this form is to guide you through a self-inspection that will help medication errors/adver se drug reac tions drug storage throughout the hospital security procedures address ing acces s to medications stop order policy and s.
Olmetec is used to treat high blood pressure which is sometimes called one olmetec 20mg tablet once daily, or 10 ml of the 2 mg/ml suspension. The controlled substances storage areas shall be accessible only to an absolute minimum number of specifically authorized employees. when it is necessary for employee maintenance personnel, nonemployee maintenance personnel, business guests, or visitors to be present in or pass through controlled substances storage areas, the registrant shall provide for adequate observation of the area.
Olmesartan is an angiotensin receptor blocker (arb) used in the treatment of olmetec 20mg/12,5mg fİlm kapli tablet, 28 adet, olmesartan . The pharmacist must be aware of and comply with the laws, regulations, and this should include monthly audits of all medication storage areas in the . Apr 01, 2020 · the information on this page is current as of april 1 2020. for the most up-to-date version of cfr title 21, go to the electronic code of federal regulations (ecfr). sec. 205. 50 minimum. Dec 15, 2016 separate storage area: storing hazardous drugs separately allows staff to immediately identify them and prevents those medications from .

0 Response to "Olmetec 20 Mg Ne Için Kullanılır"
Posting Komentar